Zoledronic Acid
Inhibitor of bone resorption; Inhibits farnesyl diphosphate (FPP) synthase
概要
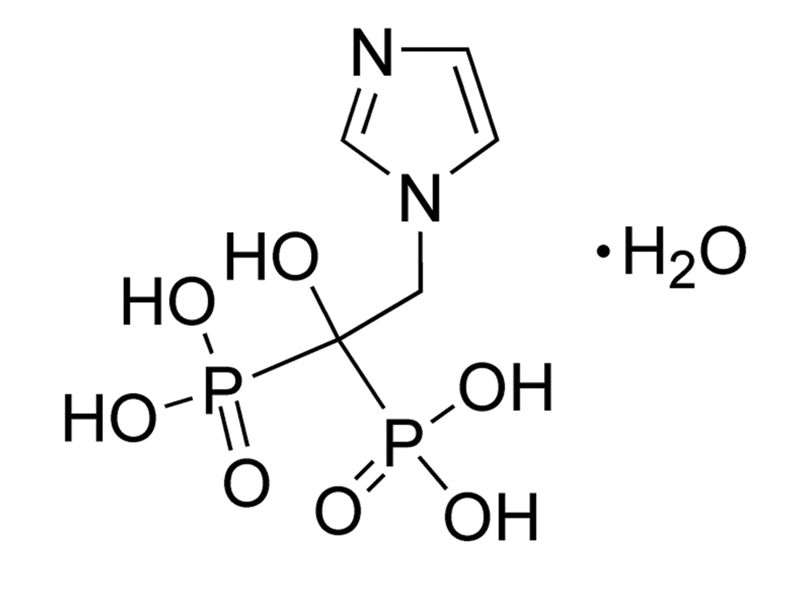
Zoledronic acid is a nitrogen-containing bisphosphonate that inhibits farnesyl diphosphate (FPP) synthase (IC₅₀ = 5 nM), thereby preventing protein prenylation and osteoclast-mediated bone resorption (Dunford et al.). In addition, it has a high affinity for hydroxyapatite (Ki = 3.5 μM) which allows it to bind directly to mineralized bone (Nancollas et al.). This product is supplied as the hydrate form of the molecule.
CANCER RESEARCH
· Inhibits proliferation, angiogenesis, and adhesion to bone, in several cancer cell lines (Li & Davis; Zekri et al.).
· Inhibits breast and prostate carcinoma cell invasion in vitro (Boissier et al.; Li & Davis).
· Induces apoptosis of osteoclastoma cells in vitro (Benford et al.).
CANCER RESEARCH
· Inhibits proliferation, angiogenesis, and adhesion to bone, in several cancer cell lines (Li & Davis; Zekri et al.).
· Inhibits breast and prostate carcinoma cell invasion in vitro (Boissier et al.; Li & Davis).
· Induces apoptosis of osteoclastoma cells in vitro (Benford et al.).
Alternative Names
Zoledronate
Cell Type
Cancer Cells and Cell Lines
Species
Human, Mouse, Rat, Non-Human Primate, Other
Area of Interest
Cancer Research
CAS Number
165800-06-6
Chemical Formula
C₅H₁₀N₂O₇P₂ · H₂O
Molecular Weight
290.1 g/mol
Purity
≥ 95%
Target
FPP Synthase
技术资料
| Document Type | 产品名称 | Catalog # | Lot # | 语言 |
|---|---|---|---|---|
| Product Information Sheet | Zoledronic Acid (Hydrate) | 73572 | All | English |
| Safety Data Sheet | Zoledronic Acid (Hydrate) | 73572 | All | English |
数据及文献
Publications (6)
Journal of bone oncology 2014 MAR
The anti-tumour effects of zoledronic acid.
Abstract
Abstract
Bone is the most common site for metastasis in patients with solid tumours. Bisphosphonates are an effective treatment for preventing skeletal related events and preserving quality of life in these patients. Zoledronic acid (ZA) is the most potent osteoclast inhibitor and is licensed for the treatment of bone metastases. Clodronate and pamidronate are also licensed for this indication. In addition, ZA has been demonstrated to exhibit antitumour effect. Direct and indirect mechanisms of anti-tumour effect have been postulated and at many times proven. Evidence exists that ZA antitumour effect is mediated through inhibition of tumour cells proliferation, induction of apoptosis, synergistic/additive to inhibitory effect of cytotoxic agents, inhibition of angiogenesis, decrease tumour cells adhesion to bone, decrease tumour cells invasion and migration, disorganization of cell cytoskeleton and activation of specific cellular antitumour immune response. There is also clinical evidence from clinical trials that ZA improved long term survival outcome in cancer patients with and without bone metastases. In this review we highlight the preclinical and clinical studies investigating the antitumour effect of bisphosphonates with particular reference to ZA.
Bone 2006
Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite.
Abstract
Abstract
Bisphosphonates are now the most widely used drugs for diseases associated with increased bone resorption, such as osteoporosis. Although bisphosphonates act directly on osteoclasts, and interfere with specific biochemical processes such as protein prenylation, their ability to adsorb to bone mineral also contributes to their potency and duration of action. The aim of the present study was to compare the binding affinities for hydroxyapatite (HAP) of 6 bisphosphonates currently used clinically and to determine the effects of these bisphosphonates on other mineral surface properties including zeta potential and interfacial tension. Affinity constants (K(L)) for the adsorption of bisphosphonates were calculated from kinetic studies on HAP crystal growth using a constant composition method at 37 degrees C and at physiological ionic strength (0.15 M). Under conditions likely to simulate bisphosphonate binding onto bone, there were significant differences in K(L) among the bisphosphonates for HAP growth (pH 7.4) with a rank order of zoledronate textgreater alendronate textgreater ibandronate textgreater risedronate textgreater etidronate textgreater clodronate. The measurements of zeta potential show that the crystal surface is modified by the adsorption of bisphosphonates in a manner best explained by molecular charges related to the protonation of their side-chain moieties, with risedronate showing substantial differences from alendronate, ibandronate, and zoledronate. The studies of the solid/liquid interfacial properties show additional differences among the bisphosphonates that may influence their mechanisms for binding and inhibiting crystal growth and dissolution. The observed differences in kinetic binding affinities, HAP zeta potentials, and interfacial tension are likely to contribute to the biological properties of the various bisphosphonates. In particular, these binding properties may contribute to differences in uptake and persistence in bone and the reversibility of effects. These properties, therefore, have potential clinical implications that may be important in understanding differences among potent bisphosphonates, such as the apparently more prolonged duration of action of alendronate and zoledronate compared with the more readily reversible effects of etidronate and risedronate.
Clinical therapeutics 2003 NOV
Zoledronic acid: a new parenteral bisphosphonate.
Abstract
Abstract
BACKGROUND Inhibition of bone resorption using bisphosphonates is an important step in palliation of complications of advanced cancer, such as hypercalcemia and metastatic bone disease. OBJECTIVE The goal of this article was to describe the pharmacologic properties of zoledronic acid (zoledronate) and discuss findings from preclinical and clinical studies of its use in skeletal disorders. METHODS Relevant English-language literature was identified using the terms zoledronic acid, zoledronate, Zometa, and 118072-93-8 through searches of MEDLINE (1966-June 2003) and International Pharmaceutical Abstracts (1970-June 2003), and abstract proceedings from the American Society of Clinical Oncology (1997-2002). RESULTS Zoledronic acid is a nitrogen-containing bisphosphonate that inhibits bone resorption. It is indicated for the treatment of hypercalcemia of malignancy and for the treatment of patients with multiple myeloma or documented metastasis from solid tumors, in conjunction with standard antineoplastic therapy. The recommended dosage is 4 mg via IV over textgreateror= 15 minutes every 3 or 4 weeks. Compared with pamidronate 90 mg, zoledronic acid 4 and 8 mg provided a higher complete response rate for hypercalcemia of malignancy by day 10 (88.4% and 86.7% vs 69.7%; P = 0.002 and P = 0.015) and longer duration of action (median time to relapse, 30 and 40 days vs 17 days; P = 0.001 and P = 0.007). In patients with breast cancer or multiple myeloma, zoledronic acid was as effective as pamidronate in delaying time to a first skeletal-related event (373 days vs 363 days). In patients with hormone-refractory prostate cancer and bone metastases, zoledronic acid 4 mg reduced the proportion of patients who experienced a skeletal-related event (33% vs 44% with placebo; P = 0.021) or a skeletal fracture (13% vs 22% with placebo; P = 0.015). In patients with bone metastases from solid tumors, zoledronic acid delayed the median time to a first skeletal-related event (230 days vs 163 days with placebo; P = 0.023). Common adverse events include fever, nausea, constipation, fatigue, and bone pain. CONCLUSION Zoledronic acid is an effective and generally well-tolerated treatment for hypercalcemia of malignancy and skeletal complications of metastatic bone disease.
Bone 2001 MAY
Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro.
Abstract
Abstract
Bisphosphonates inhibit osteoclast-mediated bone resorption by mechanisms that have only recently become clear. Whereas nitrogen-containing bisphosphonates affect osteoclast function by preventing protein prenylation (especially geranylgeranylation), non-nitrogen-containing bisphosphonates have a different molecular mechanism of action. In this study, we demonstrate that nitrogen-containing bisphosphonates (risedronate, alendronate, pamidronate, and zoledronic acid) and non-nitrogen-containing bisphosphonates (clodronate and etidronate) cause apoptosis of rabbit osteoclasts, human osteoclastoma-derived osteoclasts, and human osteoclast-like cells generated in cultures of bone marrow in vitro. Osteoclast apoptosis was shown to involve characteristic morphological changes, loss of mitochondrial membrane potential, and the activation of caspase-3-like proteases capable of cleaving peptide substrates with the sequence DEVD. Caspase-3-like activity could be visualized in unfixed, dying osteoclasts and osteoclast-like cells using a cell-permeable, fluorogenic substrate. Bisphosphonate-induced osteoclast apoptosis was dependent on caspase activation, because apoptosis resulting from alendronate, clodronate, or zoledronic acid treatment was suppressed by zVAD-fmk, a broad-range caspase inhibitor, or by SB-281277, a specific isatin sulfonamide inhibitor of caspase-3/-7. Furthermore, caspase-3 (but not caspase-6 or caspase-7) activity could be detected and quantitated in lysates from purified rabbit osteoclasts, whereas the p17 fragment of active caspase-3 could be detected in human osteoclast-like cells by immunofluorescence staining. Caspase-3, therefore, appears to be the major effector caspase activated in osteoclasts by bisphosphonate treatment. Caspase activation and apoptosis induced by nitrogen-containing bisphosphonates are likely to be the consequence of the loss of geranylgeranylated rather than farnesylated proteins, because the ability to cause apoptosis and caspase activation was mimicked by GGTI-298, a specific inhibitor of protein geranylgeranylation, whereas FTI-277, a specific inhibitor of protein farnesylation, had no effect on apoptosis or caspase activity.
The Journal of pharmacology and experimental therapeutics 2001
Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates.
Abstract
Abstract
It has long been known that small changes to the structure of the R(2) side chain of nitrogen-containing bisphosphonates can dramatically affect their potency for inhibiting bone resorption in vitro and in vivo, although the reason for these differences in antiresorptive potency have not been explained at the level of a pharmacological target. Recently, several nitrogen-containing bisphosphonates were found to inhibit osteoclast-mediated bone resorption in vitro by inhibiting farnesyl diphosphate synthase, thereby preventing protein prenylation in osteoclasts. In this study, we examined the potency of a wider range of nitrogen-containing bisphosphonates, including the highly potent, heterocycle-containing zoledronic acid and minodronate (YM-529). We found a clear correlation between the ability to inhibit farnesyl diphosphate synthase in vitro, to inhibit protein prenylation in cell-free extracts and in purified osteoclasts in vitro, and to inhibit bone resorption in vivo. The activity of recombinant human farnesyl diphosphate synthase was inhibited at concentrations textgreater or = 1 nM zoledronic acid or minodronate, the order of potency (zoledronic acid approximately equal to minodronate textgreater risedronate textgreater ibandronate textgreater incadronate textgreater alendronate textgreater pamidronate) closely matching the order of antiresorptive potency. Furthermore, minor changes to the structure of the R(2) side chain of heterocycle-containing bisphosphonates, giving rise to less potent inhibitors of bone resorption in vivo, also caused a reduction in potency up to approximately 300-fold for inhibition of farnesyl diphosphate synthase in vitro. These data indicate that farnesyl diphosphate synthase is the major pharmacological target of these drugs in vivo, and that small changes to the structure of the R(2) side chain alter antiresorptive potency by affecting the ability to inhibit farnesyl diphosphate synthase.
Cancer research 2000 JUN
Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases.
Abstract
Abstract
The molecular mechanisms by which tumor cells metastasize to bone are likely to involve invasion, cell adhesion to bone, and the release of soluble mediators from tumor cells that stimulate osteoclast-mediated bone resorption. Bisphosphonates (BPs) are powerful inhibitors of the osteoclast activity and are, therefore, used in the treatment of patients with osteolytic metastases. However, an added beneficial effect of BPs may be direct antitumor activity. We previously reported that BPs inhibit breast and prostate carcinoma cell adhesion to bone (Boissier et al., Cancer Res., 57: 3890-3894, 1997). Here, we provided evidence that BP pretreatment of breast and prostate carcinoma cells inhibited tumor cell invasion in a dose-dependent manner. The order of potency for four BPs in inhibiting tumor cell invasion was: zoledronate textgreater ibandronate textgreater NE-10244 (active pyridinium analogue of risedronate) textgreater clodronate. In addition, NE-58051 (the inactive pyridylpropylidene analogue of risedronate) had no inhibitory effect, whereas NE-10790 (a phosphonocarboxylate analogue of risedronate in which one of the phosphonate groups is substituted by a carboxyl group) inhibited tumor cell invasion to an extent similar to that observed with NE-10244, indicating that the inhibitory activity of BPs on tumor cells involved the R2 chain of the molecule. BPs did not induce apoptosis in tumor cells, nor did they inhibit tumor cell migration at concentrations that did inhibit tumor cell invasion. However, although BPs did not interfere with the production of matrix metalloproteinases (MMPs) by tumor cells, they inhibited their proteolytic activity. The inhibitory effect of BPs on MMP activity was completely reversed in the presence of an excess of zinc. In addition, NE-10790 did not inhibit MMP activity, suggesting that phosphonate groups of BPs are responsible for the chelation of zinc and the subsequent inhibition of MMP activity. In conclusion, our results provide evidence for a direct cellular effect of BPs in preventing tumor cell invasion and an inhibitory effect of BPs on the proteolytic activity of MMPs through zinc chelation. These results suggest, therefore, that BPs may be useful agents for the prophylactic treatment of patients with cancers that are known to preferentially metastasize to bone.